We are your one stop Resource for Blood-related Chromogenic products and LBC kits for Cervical Cancer

Chromogenix offers Haemostasis and fibrinolytic testing solutions based on chromogenic substrates for use in hospitals, clinical laboratories, blood banks and pharmaceutical testing. The Chromogenix product portfolio features over 30 products, including individual chromogenic substrates as well as complete kits with all reagents needed to perform specific analysis on automated instruments. Chromogenix products are manufactured, distributed and supported by Instrumentation Laboratory (www.ilww.com), a worldwide developer of in vitro diagnostics.
We from Mur & Mur, have the sole license to distribute Chromogenix Products across India and select South-East Asian Countries. We wanted to make a change with regards to testing using animal blood and we wanted to go with the best in the business. Chromogenix products are the gold standard in the industry, with FDA approvals and conforming to norms from major pharmacopoeias across the entire world. Their quality speaks for itself, and our market share across India is a testimony to that.
Chromogenic substrate for factor Xa.
Formula: Bz-IIe-Glu(γ-OR)-Gly-Arg-pNA•HCl
R=H (50%) and R=CH3 (50%)
Molecular weight: 741.3
Part Number: 82 0316 39
Chromogenic substrate for thrombin.
Formula: H-D-Phe-Pip-Arg-pNA•2HCl
Molecular weight: 625.6
Part Number: 82 0324 39
Chromogenic substrate for plasmin and streptokinase-activated plasminogen.
Formula: H-D-Val-Leu-Lys-pNA•2HCl
Molecular weight: 551.6
Part Number: 82 0332 39
Chromogenic substrate for t-PA and a broad spectrum of other serine proteases.
Formula: H-D-Ile-Pro-Arg-pNA•2HCl
Molecular weight: 577.6
Part Number: 82 0852 39
Chromogenic substrate for plasma kallikrein and factor XIIa.
Formula: H-D-Pro-Phe-Arg-pNA•2HCl
Molecular weight: 611.6
Part Number: 82 0340 39
Chromogenic substrate for activated protein C and factor XIa.
Formula: pyroGlu-Pro-Arg-pNA•HCl
Molecular weight: 539.0
Part Number: 82 1090 39
Chromogenic substrate for plasmin and streptokinase-activated plasminogen.
Formula: pyroGlu-Phe-Lys-pNA•HCl
Molecular weight: 561.0
Part Number: 82 2254 39
Chromogenic substrate for factor Xa.
Formula: Z-D-Arg-Gly-Arg-pNA•2HCl
Molecular weight: 714.6
Part Number: 82 1413 39
Composition and purity: Lyophilised powder prepared from human plasma after affinity chromatography on heparin-Sepharose gel.
Pure preparation. Does not contain stabiliser.
Note: This preparation is not a standard.
Package 1x25 IU
Part Number: 81 0796 39
Composition and purity: Lyophilised powder prepared from human plasma after affinity chromatography on heparin-Sepharose gel. Contains human albumin as a stabiliser.
Note: This preparation is not a standard.
Included in: COATEST® HEPARIN
Package 10x10 IU
Part Number: 82 0720 39
Composition and purity: Lyophilised powder prepared from bovine plasma and purified by barium citrate adsorption and liquid chromatography. Activation is performed by matrix bound activator from Russell’s Viper Venom. Contains buffer salts, albumin and polyethyleneglycol. The activity (71 nkat) is determined with the substrate S-2222.
Included in: COATEST® HEPARIN
Package 10x71 nkat
Part Number: 82 0985 39
Composition: Lyophilised citrated, stabilised human plasma prepared from pools of plasma collected from healthy donors.
Application: Quality control of Coatest® APCTM Resistance and Coatest® APCTM Resistance V.
Package: 5x1 ml
Part Number: 82 2650 63
Composition: Lyophilised citrated, stabilised human plasma prepared from pools of plasma collected from donors carrying the Factor V:Q506 mutation.
Application: Quality control of Coatest® APCTM Resistance and Coatest® APCTM Resistance V.
Package: 5x1 ml
Part Number: 82 2668 63
An APTT-based assay for the detection of the APC resistance phenotype, i.e. the poor anticoagulant response to activated protein C (APC). The test result (APC ratio) gives an estimation of the anticoagulant function in vivo and provides information on the thrombotic risk associated with inherited and acquired APC resistance.
Measurement Principle: Plasma is incubated with the APTT reagent for a standard period of time. Coagulation is initiated by the addition of CaCl2 in the absence and presence of APC and the time for clot formation is recorded.
Determinations: Automated methods 80-160
Part Number: 82 2643 63
A chromogenic kit for the determination of heparin and low molecular weight heparin in human plasma.
Measurement Principle :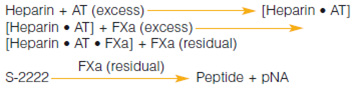
Determinations:
Test tube method 100
Microplate method 400
Automated methods up to 285
Part Number: 25 5539 63
A chromogenic kit for the determination of protein C activity in human plasma. No influence of heparin levels up to 3 IU/ml.
Measurement Principle :
Determinations:
Automated methods up to 180
Microplate method 288
Test tube method 72
Part Number: 82 2098 63
Automated latex ligand immunoassay for the quantitative determination of free Protein S (PS) in human citrated plasma on automated instruments. Two forms of Protein S are present in plasma: free Protein S (40%), and Protein S linked to the complement C4b-binding protein (C4BP) (60%).
Only free Protein S has functional cofactor activity.
Measurement Principle: The presence of free Protein S in the sample is measured as the increase of turbidity produced by the agglutination of two latex reagents. Purified C4BP adsorbed onto the first latex reagent reacts with a high affinity for free Protein S of patient plasma in the presence of Ca 2+ ions. The free Protein S adsorbed on the C4BP latex triggers the agglutination reaction with the second latex reagent, which is sensitized with a monoclonal antibody directed against human Protein S. The degree of agglutination will be directly proportional to the free Protein S concentration in the test sample and is determined by measuring the decrease of the transmitted light at 405 nm caused by the aggregates.
Determinations:
Approximately 75 Tests
Part Number: 82 4003 63
A chromogenic kit for the determination of factor VII activity in human plasma. Not affected by preactivation of factor VII.
Measurement Principle : 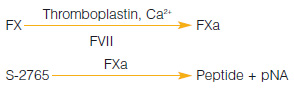
Determinations:
Test tube method 30
Microplate method 120
Automated methods up to 120
Part Number: 82 1900 63
A chromogenic kit for the determination of factor VIII activity in human plasma, blood fractions and purified preparations. Fulfills the requirements of the European Pharmacopoeia for factor VIII concentrate testing.
Measurement Principle : 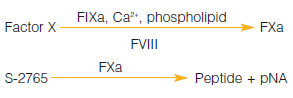
Determinations:
Test tube method 30
Microplate method 120
Automated methods up to 100
Part Number: 82 2585 63
The classic chromogenic kit for the determination of factor VIII activity in human plasma, blood fractions and purified preparations.
Measurement Principle : 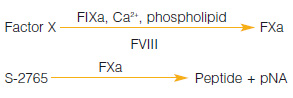
Determinations:
Test tube method 60
Microplate method 240
Automated methods up to 200
Part Number: 82 4086 63
A chromogenic kit for the determination of factor VIII activity in human plasma, blood fractions and purified preparations.
Suitable for low volume testing.
Measurement Principle : 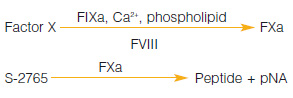
Determinations:
Test tube method 4x20
Microplate method 240
Automated methods up to 200
Part Number: 82 4094 63
Automated latex enhanced immunoassay for the quantitative determination of von Willebrand Factor Antigen (VWF:Ag) in human citrated plasma on IL Coagulation Systems.
The diagnosis of von Willebrand disease (VWD), probably the most common congenital bleeding disorder, requires a number of special tests at the laboratory level.1 Among them, VWF:Ag determination is essential and must be performed on every patient to reach a proper diagnosis.1 Depending upon these laboratory findings, VWD is classified into type 1 (the most frequent form being 70-80% of VWD), type 2 or type 3 (1 to 3% of VWD) groups.2,3 Type 1 shows a reduction of VWF although its structure and functionality is normal. In type 3, VWF is almost absent in plasma. In type 2 the quantity of VWF in plasma may be normal or slightly reduced but its molecular structure and its functionality is abnormal. Type 2 may be further characterized into subtypes by multimeric structure analysis of VWF. Apart from the above described inherited VWD, acquired VWD due to autoantibodies or to various disease states resulting in low rates of VWF synthesis has been reported. On the other side, chronic or acute inflammatory diseases or processes involving damage of the vascular endothelium yield abnormally high concentrations of VWF.4 The VWF:Ag kit is a latex particle enhanced immunoturbidimetric assay to quantify VWF:Ag in plasma. When a plasma containing VWF:Ag is mixed with the Latex Reagent and the Reaction Buffer included in the kit, the coated latex particles agglutinate. The degree of agglutination is directly proportional to the concentration of VWF:Ag in the sample and is determined by measuring the decrease of transmitted light caused by the aggregates.
The von Willebrand Factor Antigen kit consists of:
Unopened reagents are stable until the expiration date shown on the vial when stored at 2-8°C. Opened reagents are stable 3 months at 2-8°C in the original vial or 1 week at 15°C on the ACL Elite®/Elite® Pro and ACL TOP® Family/ACL TOP Family 50 Series†. Do not freeze. For optimal stability remove reagents from the system and store them at 2-8°C in the original vial. Low relative humidity is associated with increased evaporation of uncapped reagents, which may decrease on-board stability. For optimum on-board stability, laboratory temperature and humidity